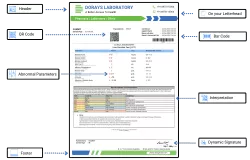
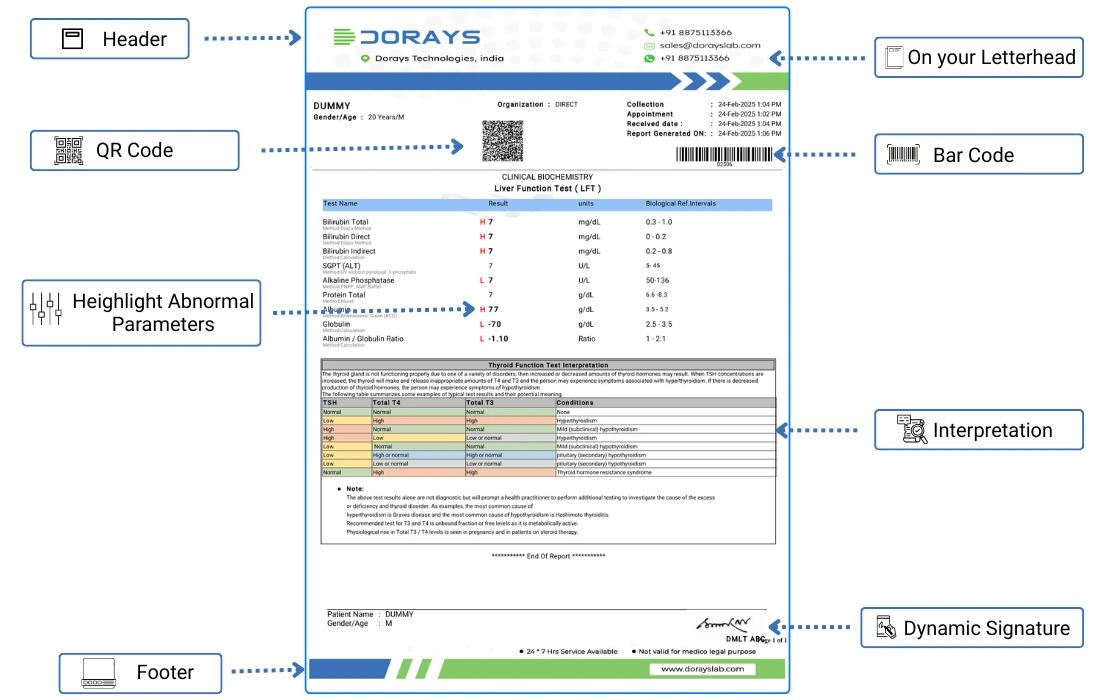
Structure of the DoraysLIS Report
SUPPORT
Frequently Asked Questions
We design our products in such a way that makes software purchase decisions for our customers more value based than traditional software companies.
Yes. With DoraysLIS, you can customize the report templates with your logo, color scheme, fonts, address, header/footer content, and preferred layout to match your lab needs and company requirements.
Essential identifiers included in the report templates are:
- Patient's unique identification number
- Patient details
- Lab registration number
- Date and time of sample collection
- Specimen type and quality
- Date and time of report generation
- Referral doctor’s details
- Hospital/clinic information (if applicable)
- Test method
- normal range based on gender
- Interpretation/contextual remarks/recommended action
- Digital signature
Absolutely, DoraysLIS provides 140+ specialized templates for various departments, including pathology, biochemistry, hematology, radiology, and microbiology. There are also miscellaneous templates for uncategorized tests.
You can also assign different report templates to specific doctors, departments, or test types to maintain clarity and consistency.
Rest assured, our report formats are designed to comply with the Indian Council of Medical Research (ICMR) standards and guidelines.
DoraysLIS is a secure cloud-based lab management system. Therefore, all our reports go through several layers of protection, including:
- HIPAA and Indian medical data protection guidelines compliance
- Secure report access protocols
- Patient data anonymization technology
- QR Code integration
- Digital signature and encryption
- In-built restrictions on report sharing
As an advanced lab information management system, DoraysLIS Report Formats offer:
- QR code/ barcode readability
- Multiple language support
- Compatibility with different systems
- Mobile and web-based report viewing options
- PDF and MS Word formats
Yes. You can instantly share reports via email, WhatsApp, or SMS directly from the LIS without manual intervention. This reduces time and effort.
Yes. DoraysLIS allows consolidated reporting where you can create summary reports for corporate clients, health camps, or group testing conditions.
Yes, all our reports will be securely saved in our dedicated cloud server. You can search, retrieve, download, and resend reports anytime from anywhere with your secure login credentials.
Trusted By 1100+ Businesses Globally
Exciting Updates at DoraysLis
Discover the latest advancements, services, and features we're offering to enhance your experience at DoraysLis. We're constantly evolving to provide you with the best diagnostic services, cutting-edge technology, and expert care. Stay tuned for our new offerings and improvements that will make your visits more efficient and comprehensive.